Have you received REBYOTA to keep recurrent C. diff from coming back? SHARE YOUR STORY>
What is the gut microbiome?
Your gut is home to trillions of different, living microbes all contributing to your health and wellness. This is called your gut microbiome. Together, these microbes—including both good and bad bacteria—can do many things, including help protect against infections.1-3
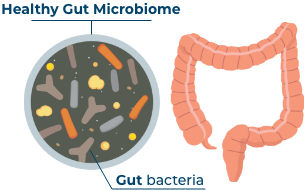
What does a healthy gut microbiome look like?
A healthy gut microbiome is a complex mix of many different bacteria, made up mostly of 2 categories known as Bacteroidetes and Firmicutes, working together in balance to maintain gut health by1-3:
- Regulating immune function4,5
- Protecting against inflammation6-11
When the volume and mix of bacteria are compromised, the microbiome is disrupted.2,12
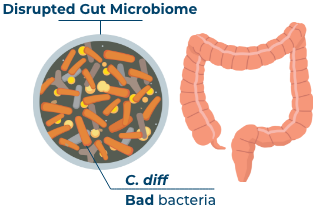
What happens when a healthy gut is disrupted?
If this important mixture of bacteria (including Bacteroidetes and Firmicutes) is disrupted, it can place you at risk for infection. One of the infections is called C. difficile (or C. diff).12
C. diff infection and its recurrence
C. diff is a bacteria that can cause an infection in the gut. Symptoms can range from diarrhea to life-threatening damage to the colon.12 Sometimes, a C. diff infection can come back more than once. This is called a recurrence.2,12 If left untreated, it too can cause severe illness, and even death.12
Microbiome disruption increases the risk of C. diff infection recurrence.12,13
- Antibiotics treat C. diff infection but can also be a leading cause of C. diff recurrence12,13
- Probiotics are recommended only to keep a balanced gut healthy and are not recommended to treat or prevent C. diff.
Some believe probiotics can negatively affect gut health after antibiotics14-16

Up to 35% of people who get a C. diff infection may have a recurrence17,18

Up to 65% of people who experience a recurrence may have a second or third or more19,20

Learn About REBYOTA
REBYOTA is the first and only single-dose, FDA approved microbiome-based treatment to keep recurrent C. diff infection from coming back, after you’ve already taken antibiotics.23


If you’re ready to talk to a doctor, this customized discussion guide is a great way to prepare.
Sign up for more information
Receive additional information and resources to help you through your C. diff infection journey.
References
- Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24(4):392-400.
- Antharam VC, Li EC, Ishmael A, et al. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol. 2013;51(9):2884-2892.
- Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823-1836.
- Wexler AG, Goodman AL. An insider’s perspective: Bacteroides as a window into the microbiome. Nat Microbiol. 2017;2:17026.
- Li X, Kang Y, Huang Y, et al. A strain of Bacteroides thetaiotaomicron attenuates colonization of Clostridioides difficile and affects intestinal microbiota and bile acids profile in a mouse model. Biomed Pharmacother. 2021;137:111290.
- Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A. 2004;101(28):10398-10403.
- El Aidy S, van Baarlen P, Derrien M, et al. Temporal and spatial interplay of microbiota and intestinal mucosa drive establishment of immune homeostasis in conventionalized mice. Mucosal Immunol. 2012;5(5):567-579.
- Lawley TD, Walker AW. Intestinal colonization resistance. Immunology. 2013;138(1):1-11.
- Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105(43):16731-16736.
- Willing BP, Dicksved J, Halfvarson J, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139(6):1844-1854.e1.
- Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275-1283.
- Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Therap Adv Gastroenterol. 2013;6(1):53-68.
- McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48.
- Cordaillat-Simmons M, Rouanet A, Pot B. Live biotherapeutic products: the importance of a defined regulatory framework. Exp Mol Med. 2020;52(9):1397-1406.
- Kelly CR, Fischer M, Allegretti JR, et al. ACG Clinical Guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. 2021;116(6):1124-1147.
- Suez J, Zmora N, Ziblerman-Schapira G, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406-1423.e16.
- Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
- Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
- Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
- Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2:16020.
- Nelson WW, Scott TA, Boules M, et al. Health care resource utilization and costs of recurrent Clostridioides difficile infection in the elderly: a real-world claims analysis. J Manag Care Spec Pharm. 2021;27(7):828-838.
- Feuerstadt P, Boules M, Stong L, et al. Clinical complications in patients with primary and recurrent Clostridioides difficile infection: a real-world data analysis. SAGE Open Med. 2021;9:2050312120986733.
- REBYOTA. Prescribing Information. Parsippany, NJ: Ferring Pharmaceuticals Inc; 2022.
Please see Important Safety Information and full Prescribing Information
INDICATION
REBYOTA (fecal microbiota, live – jslm) is indicated for the prevention of recurrence of Clostridioides difficile (C. diff) infection in individuals 18 years of age and older, following antibiotic treatment for recurrent C. diff infection.
Limitation of Use
REBYOTA is not indicated for the treatment of C. diff infection.
Important Safety Information
- You should not receive REBYOTA if you have a history of a severe allergic reaction (e.g., anaphylaxis) to REBYOTA or any of its components.
- You should report to your doctor any infection you think you may have acquired after administration.
- REBYOTA may contain food allergens.
- Most common side effects may include stomach pain (8.9%), diarrhea (7.2%), bloating (3.9%), gas (3.3%), and nausea (3.3%).
- REBYOTA has not been studied in patients below 18 years of age.
- Clinical studies did not determine if adults 65 years of age and older responded differently than younger adults.
You are encouraged to report negative side effects of prescription drugs to FDA. Visit www.FDA.gov/medwatch or call 1-800-332-1088.
Please click to see the full Prescribing Information



